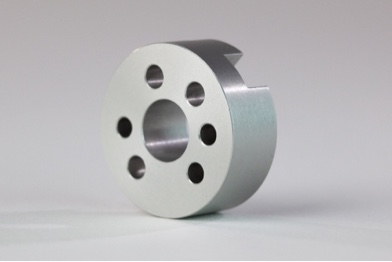
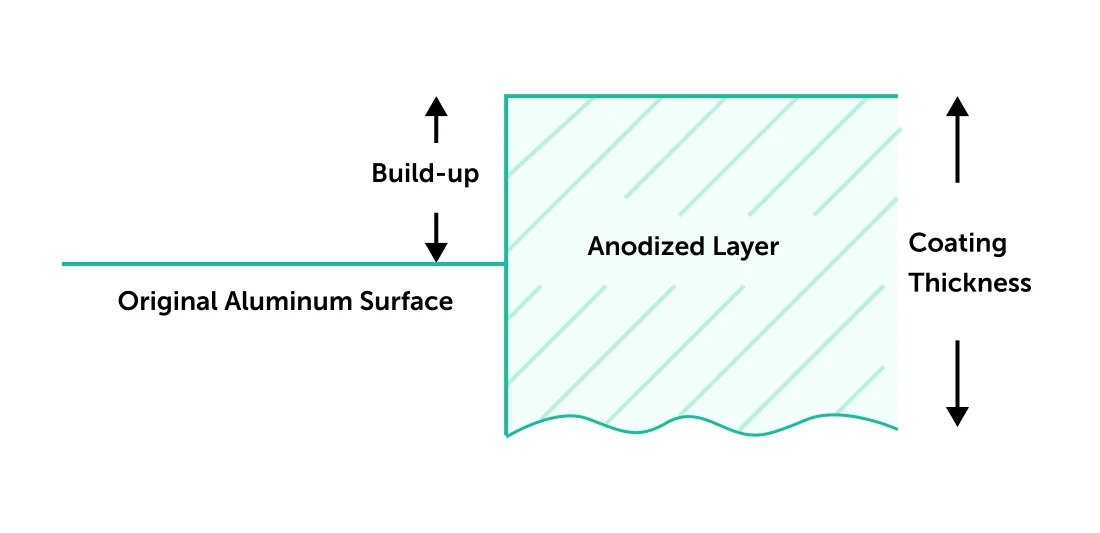
Anodizing is an electrolytic passivation process that grows the natural oxide layer on aluminum parts for protection from wear and corrosion, as well as for cosmetic effects. It is a conversion coating, similar to Alodine, meaning that the surface of the aluminum recedes dimensionally before the protective oxide layer is built up. After the process is complete, the oxide layer is integral to the aluminum substrate below, which means it won’t chip or flake.
The name anodizing comes from the fact that the treated part forms an anode (positive electrode) in an electrical circuit. During this process, the part to be anodized is hung on a conductive rack and submerged in an electrolytic solution, where a direct current of electricity is introduced. While the acidity of the solution dissolves the oxide layer of the part, the electric current releases oxygen at its surface, which builds up a protective layer of aluminum oxide. By balancing dissolve rate with build-up rate, the oxide layer forms with nanopores, allowing continued growth of the coating beyond what is naturally possible.
The final steps of the anodizing process involve sealing the nanopores. Otherwise, they are the perfect passageways for corrosion initiation! Just before sealing, however, they are sometimes filled with other corrosion inhibitors or colored dyes for cosmetic purposes. After the process is complete, the coating will be 0.0002-0.0012” in thickness, in accordance with the common engineering spec MIL-A-8625 Type II.